Given the high occurrence of pain in patients with spasticity, managing it should be an integral part of the patient treatment plan2
Prevalence of pain in patients with spasticity
Cerebral Palsy
Spinal Cord Injury
Traumatic Brain Injury
Stroke
Cryoneurolysis:
An innovative approach to pain management
iovera° provides targeted pain relief
without permanently
damaging nerves, unlike other treatment options3
Comparison of nerve treatments
-
-140°C
- -88°C
-
-
Cryoablation provides irreversible nerve degeneration via liquid nitrogen4
-140°C
-
Cryoneurolysis provides immediate treatment effect without permanently destroying nerves5
-88°C
-
-
- 40°C
- 60°C to 80°C
-
-
Pulsed RF
40°C -
RF risks neuroma formation postdenervation, neuritis, and injury to nearby tissue and vessels5
-
Cooled/Conventional RF
60°C to 80°C
-
The iovera°
system:
Targeted cryoneurolysis for immediate and long-lasting pain
management
- Immediate, safe, and effective6
- Drug-free and non-systemic3
- Handheld device providing targeted, long-lasting pain relief through cryoneurolysis6
- Creates a long-lasting nerve block by treating peripheral nerves to temporarily stop pain signals6
- Significantly improved pain relief and reduced opioid use through 90 days, along with significant improvement in flexion and range of motion through 12 weeks6-8
- Treatment can be performed under ultrasound guidance9
Designed for relief
1
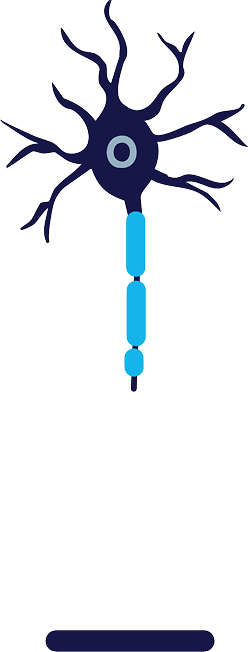
Treatment
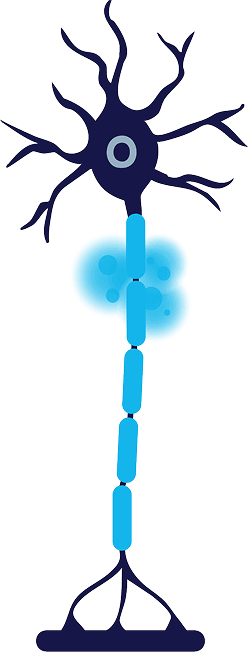
Cold zone is created around the targeted nerve, reaching a temperature of -88°C
2
Degeneration
Cold zone causes degeneration of axon and myelin sheath, temporarily blocking nerve signals
3
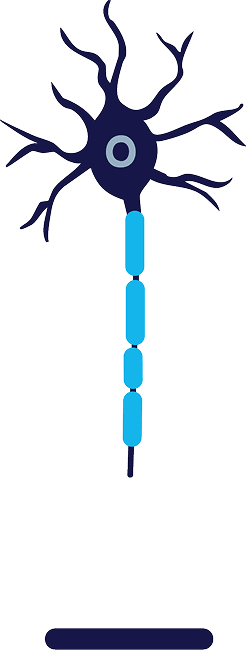
Regeneration
Following treatment,
the axon
regenerates at
about 1 to 3 mm/day1
4
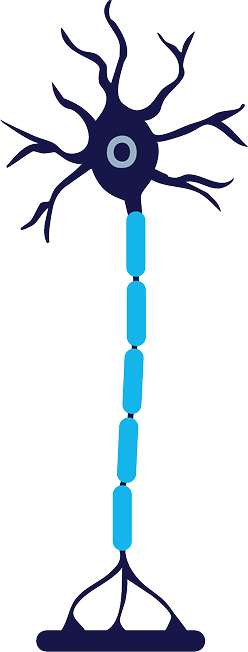
Reinnervation
The axon and myelin sheath are fully regenerated, and nerve signaling is restored
See how your peers use iovera° for pain associated with spasticity

Upper Extremity Pain Associated With Spasticity
A case report featuring Dr. Winston focused on a treatment performed with iovera° for pain associated with spasticity.

Lower Extremity Pain Associated With Spasticity
A case report featuring Dr. Boissonnault focused on a treatment performed with iovera° for pain associated with spasticity.
RF=radiofrequency.
- References:
- 1. Scobie J, Winston P. Case report: perspective of a caregiver on functional outcomes following bilateral lateral pectoral nerve cryoneurotomy to treat spasticity in a pediatric patient with cerebral palsy. Front Rehabil Sci. 2021;2:19054. doi.org/10.3389/fresc.2021.719054.
- 2. Shaikh A, Phadke CP, Ismail F, Boulias C. Relationship between botulinum toxin, spasticity, and pain: a survey of patient perception. J Neurol Sci. 2016;43(2):311-315.
- 3. Ilfeld BM, Preciado J, Trescot AM. Novel cryoneurolysis device for the treatment of sensory and motor peripheral nerves. Expert Rev Med Devices. 2016;13(8):713-725.
- 4. Zhou L, Craig J, Parekh N. Current concepts of neurolysis and clinical applications. J Analgesics. 2014;2(1):16-22.
- 5. Barnard D. The effects of extreme cold on sensory nerves. Ann R Coll Surg Engl. 1980;62(3):180-187.
- 6. Radnovich R, Scott D, Patel AT, et al. Cryoneurolysis to treat the pain and symptoms of knee osteoarthritis: a multicenter, randomized, double-blind, sham-controlled trial. Osteoarthritis Cartilage. 2017;25(8):1247-1256.
- 7. Plessl D, Salomon B, Haydel A,
Leonardi C, Bronstone A, Dasa V. Rapid versus standard recovery protocol is
associated with improved recovery of range of motion 12 weeks after total
knee arthroplasty. J Am Acad Orthop Surg. 2020;28(21):e962-e968.
- 8. Dasa V, Lensing G, Parsons M. Harris
J, Volaufova J, Bliss R. Percutaneous freezing of sensory nerves prior to
total knee arthroplasty. Knee. 2016;23(3):523-528.
- 9. Data on file. Pacira BioSciences, Inc.; 2022.
Indication
The iovera° system is used to destroy tissue during surgical procedures by applying freezing cold. It can also be used to produce lesions in peripheral nervous tissue by the application of cold to the selected site for the blocking of pain. It is also indicated for the relief of pain and symptoms associated with osteoarthritis of the knee for up to 90 days. The iovera° system is not indicated for treatment of central nervous system tissue.
Important Safety Information
Contraindications
The iovera° system is contraindicated for use in patients with the following:
- Cryoglobulinemia, paroxysmal cold hemoglobinuria, cold urticaria, Raynaud’s disease, and open and/or infected wounds at or near the treatment site
Potential Complications
As with any surgical treatment that uses needle-based therapy and local anesthesia, there is a potential for site-specific reactions, including, but not limited to:
- Ecchymosis, edema, erythema, local pain and/or tenderness, and localized dysesthesia
Proper use of the device as described in the User Guide can help reduce or prevent the following complications:
- At the treatment site(s): injury to the skin related to application of cold or heat, hyper- or hypopigmentation, and skin dimpling
- Outside the treatment site(s): loss of motor function
For more safety information please visit: https://www.iovera.com/safety
Indication
The iovera° system is used to destroy tissue during surgical procedures by applying freezing cold. It can also be used to produce lesions in peripheral nervous tissue by the application of cold to the selected site for the blocking of pain.
Important Safety Information




